Marcela Rodriguez, Ph.D.
Associate Professor of Medicine
rodrigg2@njms.rutgers.edu | View CV
+1-973-854-3261
NEWS:
Our latest publication:
Sharma N, Sharma N, Biswas A, Gupta S, Behura A, Rodriguez GM (2025) Iron-restricted Mycobacterium tuberculosis exports pathogenicity factors packed in extracellular vesicles. PLoS One 20: e0324919. PMI: 40445943
The studies conducted in the Rodriguez laboratory are focused in the role of metal homeostasis in pathogenesis of tuberculosis. We are interested in the effects of metal ion restriction resulting from nutritional immunity on the pathogenesis of Mycobacterium tuberculosis.
Like most living organisms M. tuberculosis requires iron as redox cofactor of enzymes involved in vital cellular functions that range from respiration to DNA replication. Due to the poor solubility of ferric iron in aerobic conditions, free iron is not found in the mammalian host. A successful pathogen has to be able to scavenge iron to proliferate and cause disease. However, excess iron is potentially toxic because it catalyzes the generation of harmful reactive oxygen species. Therefore, all aerobic organisms must tightly control intracellular iron levels. In our scientific approach we combine in vitro, ex vivo and in vivo approaches to define the role of metal homeostasis in pathogenesis of tuberculosis. Our scientific goal is to apply the knowledge derived from our basic studies to the development of new therapeutic or preventive tools against M. tuberculosis. Our current research interests include:
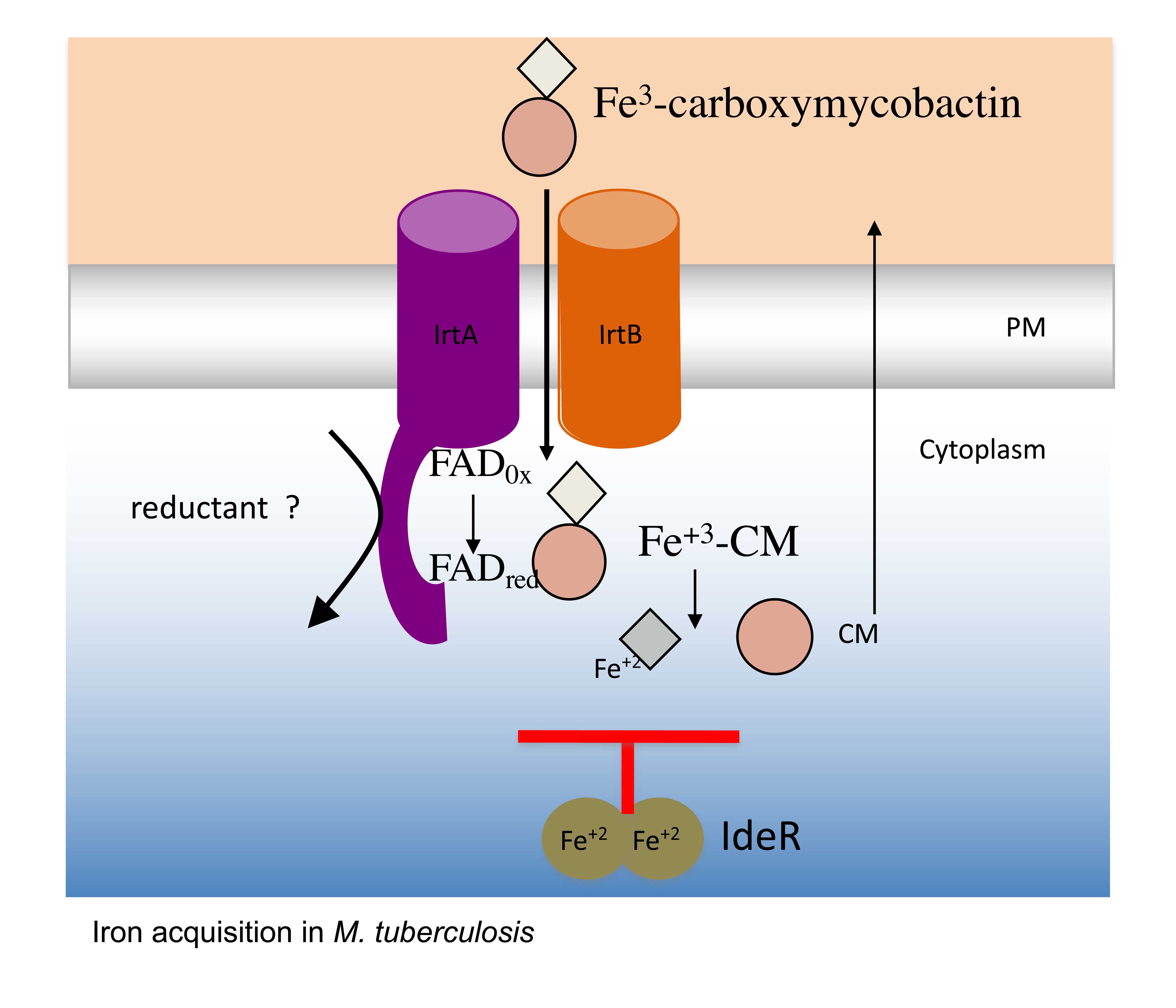
The molecular mechanisms involved in essential metal acquisition by M. tuberculosis: Our current studies focus in defining the molecular machinery employed by M. tuberculosis to acquire essential metals particularly iron and manganese to proliferate in the host, as these molecules are potential targets for therapeutic intervention (Figure 1).
Targeting regulatory mechanisms controlling metal homeostasis in M. tuberculosis. Iron is an essential micronutrient required by M. tuberculosis to establish a productive infection. However, excess iron can be very toxic for most aerobic organisms. Therefore, iron dependent cells must balance intracellular iron levels. Evidence from animal studies indicates that the ability to maintain iron homeostasis is essential for M. tuberculosis to proliferate and cause disease. M. tuberculosis depends on the global transcriptional regulator, IdeR to maintain iron homeostasis. IdeR controls iron metabolism and is essential for M. tuberculosis’ virulence (figure 2). We are currently investigating diverse therapeutic strategies to interfere with IdeR function to control M. tuberculosis replication.
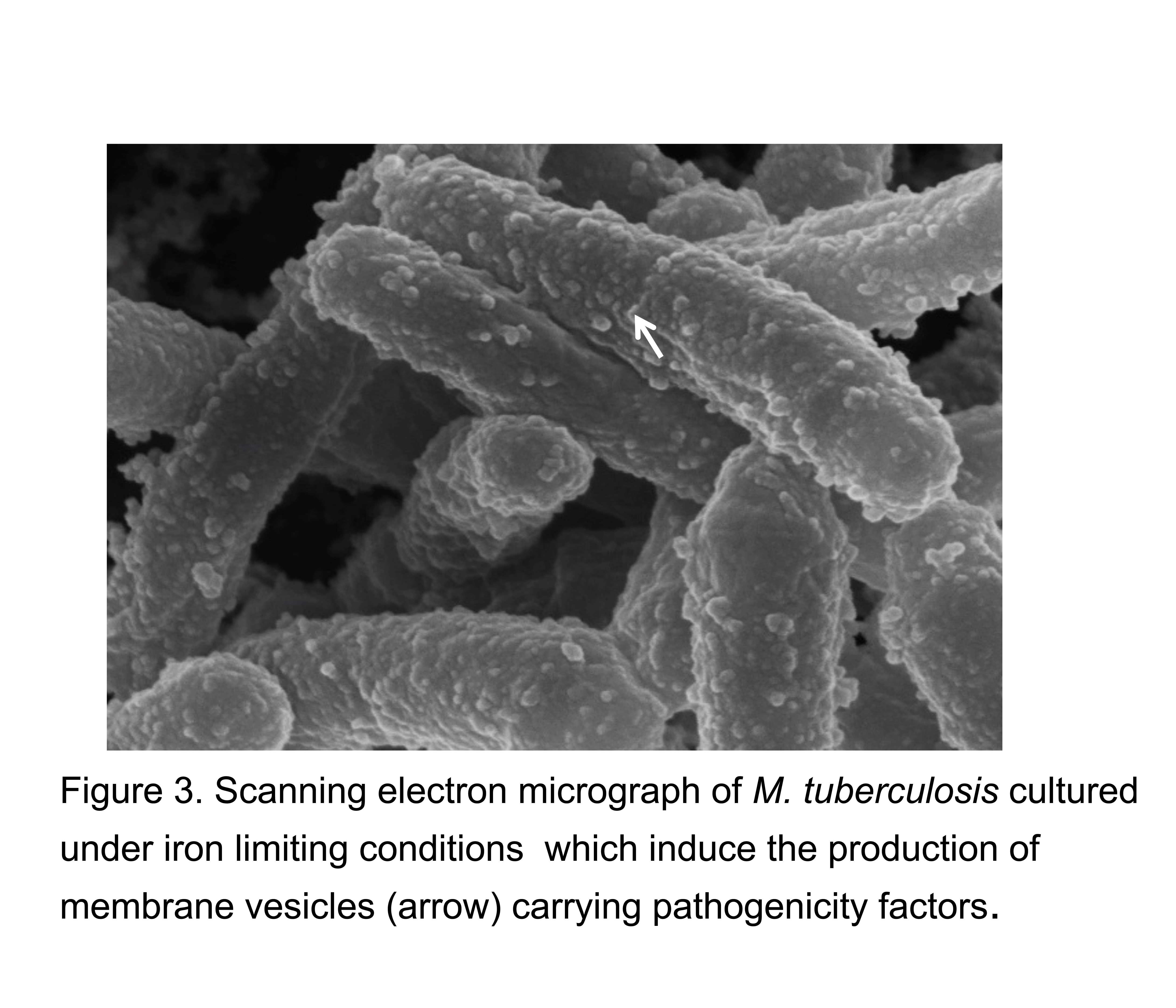
Mechanisms and regulation of membrane vesicle production in mycobacteria. We discovered that in response to iron limitation, a condition encountered by mycobacteria in the host, M. tuberculosis upregulates the production of membrane vesicles, which have since emerged as a novel mechanism used by M. tuberculosis to evade and modulate the immune response (figure 3). Our current studies combine genetic, biochemical, microscopy and microbiological approaches to better understand vesicle formation and its regulation in mycobacteria.
Metal homeostasis and persistence of M. tuberculosis. Our studies have shown that the ultimate response of M.tuberculosis to iron-deprivation is survival without replication, upregulation of host survival factors and antibiotic tolerance. We investigate the mechanisms that enable M.tuberculosis to persist without iron to better understand the physiology of bacteria that may maintain the characteristic chronicity of TB.
Sharma N, Sharma N, Biswas A, Gupta S, Behura A, Rodriguez GM (2025) Iron-restricted Mycobacterium tuberculosis exports pathogenicity factors packed in extracellular vesicles. PLoS One 20: e0324919. PMI: 40445943
Gupta S, Bhagavathula M, Sharma V, Sharma N, Sharma N, Biswas A, Palacios A, Salgueiro V, Lavin JL, Dogra N, Salgame P, Prados-Rosales R, Rodriguez GM (2023) Dynamin-like proteins mediate extracellular vesicle secretion in Mycobacterium tuberculosis. EMBO Rep: e55593. PMI: 37079766
Rodriguez GM, Sharma N, Biswas A, Sharma N (2022) The Iron Response of Mycobacterium tuberculosis and Its Implications for Tuberculosis Pathogenesis and Novel Therapeutics. Front Cell Infect Microbiol 12: 876667. PMI: 35646739
Li M, Yamada Y, Rodriguez GM, Dick T, Go ML (2022) Redox Cycling Dioxonaphthoimidazoliums Disrupt Iron Homeostasis in Mycobacterium bovis Bacillus Calmette-Guerin. Microbiol Spectr: e0197022. PMI: 36377959
Palacios A, Gupta S, Rodriguez GM, Prados-Rosales R (2021) Extracellular vesicles in the context of Mycobacterium tuberculosis infection. Mol Immunol 133: 175-181. PMI: 33743266
Gupta S, Palacios A, Khataokar A, Weinrick B, Lavín JL, Sampedro L, Gil D, Anguita J, Menendez MC, García MJ, Dogra N, Neiditch MB, Prados-Rosales R, Rodríguez GM (2020) Dynamin-like proteins are essential for vesicle biogenesis in Mycobacterium tuberculosis. bioRxiv: 2020.2001.2014.906362.
Gupta S, Rodriguez GM (2019) Isolation and Characterization of Extracellular Vesicles Produced by Iron-limited Mycobacteria. JoVE: e60359.
Visit www.jove.com to view the video associated with the article.
Kolloli A, Singh P, Rodriguez GM, Subbian S (2019) Effect of Iron Supplementation on the Outcome of Non-Progressive Pulmonary Mycobacterium tuberculosis Infection. J Clin Med 8. PMI: 31382404
Gupta S, Rodriguez GM (2018) Mycobacterial extracellular vesicles and host pathogen interactions. Pathog Dis 76. PMI: 29722822
Kurthkoti K, Amin H, Marakalala MJ, Ghanny S, Subbian S, Sakatos A, Livny J, Fortune SM, Berney M, Rodriguez GM (2017) The Capacity of Mycobacterium tuberculosis To Survive Iron Starvation Might Enable It To Persist in Iron-Deprived Microenvironments of Human Granulomas. MBio 8. PMI: 28811344
Rodriguez GM, Prados-Rosales R (2016) Functions and importance of mycobacterial extracellular vesicles. Appl Microbiol Biotechnol 100: 3887-3892. PMI: 27020292
Subbian S, Pandey R, Soteropoulos P, Rodriguez GM (2015) Vaccination with an Attenuated Ferritin Mutant Protects Mice against Virulent Mycobacterium tuberculosis. J Immunol Res 2015: 385402. PMI: 26339659
Pandey R, Russo R, Ghanny S, Huang X, Helmann J, Rodriguez GM (2015) MntR(Rv2788): a transcriptional regulator that controls manganese homeostasis in Mycobacterium tuberculosis. Mol Microbiol. PMI: 26337157
Kurthkoti K, Tare P, Paitchowdhury R, Gowthami VN, Garcia MJ, Colangeli R, Chatterji D, Nagaraja V, Rodriguez GM (2015) The mycobacterial iron-dependent regulator IdeR induces ferritin (bfrB) by alleviating Lsr2 repression. Mol Microbiol. PMI: 26268801
Prados-Rosales R, Weinrick BC, Pique DG, Jacobs WR, Jr., Casadevall A, Rodriguez GM (2014) Role for Mycobacterium tuberculosis Membrane Vesicles in Iron Acquisition. J Bacteriol 196: 1250-1256. PMI: 24415729
Pandey R, Rodriguez GM (2014) IdeR is required for iron homeostasis and virulence in Mycobacterium tuberculosis. Mol Microbiol 91: 98-109. PMI: 24205844
Serafini A, Pisu D, Palu G, Rodriguez GM, Manganelli R (2013) The ESX-3 secretion system is necessary for iron and zinc homeostasis in Mycobacterium tuberculosis. PLoS One 8: e78351. PMI: 24155985
Santhanagopalan SM, Rodriguez GM (2012) Examining the role of Rv2895c (ViuB) in iron acquisition in Mycobacterium tuberculosis. Tuberculosis (Edinb) 92: 60-62. PMI: 22015175
Pandey R, Rodriguez GM (2012) A ferritin mutant of Mycobacterium tuberculosis is more susceptible to killing by antibiotics and is unable to establish a chronic infection in mice. Infect Immun. PMI: 22802345
Madigan CA, Cheng TY, Layre E, Young DC, McConnell MJ, Debono CA, Murry JP, Wei JR, Barry CE, 3rd, Rodriguez GM, Matsunaga I, Rubin EJ, Moody DB (2012) Lipidomic discovery of deoxysiderophores reveals a revised mycobactin biosynthesis pathway in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 109: 1257-1262. PMI: 22232695
Ryndak MB, Wang S, Smith I, Rodriguez GM (2010) The Mycobacterium tuberculosis high-affinity iron importer, IrtA, contains an FAD-binding domain. J Bacteriol 192: 861-869. PMI: 19948799
Janagama HK, Senthilkumar TM, Bannantine JP, Rodriguez GM, Smith I, Paustian ML, McGarvey JA, Sreevatsan S (2009) Identification and functional characterization of the iron-dependent regulator (IdeR) of Mycobacterium avium subsp. paratuberculosis. Microbiology 155: 3683-3690. PMI: 19684064
Rodriguez GM, Gardner R, Kaur N, Phanstiel Ot (2008) Utilization of Fe3+-acinetoferrin analogs as an iron source by Mycobacterium tuberculosis. Biometals 21: 93-103. PMI: 17401548
Rao PK, Rodriguez GM, Smith I, Li Q (2008) Protein dynamics in iron-starved Mycobacterium tuberculosis revealed by turnover and abundance measurement using hybrid-linear ion trap-Fourier transform mass spectrometry. Anal Chem 80: 6860-6869. PMI: 18690695
Maciag A, Dainese E, Rodriguez GM, Milano A, Provvedi R, Pasca MR, Smith I, Palu G, Riccardi G, Manganelli R (2007) Global analysis of the Mycobacterium tuberculosis Zur (FurB) regulon. J Bacteriol 189: 730-740. PMI: 17098899
Rodriguez GM, Smith I (2006) Identification of an ABC transporter required for iron acquisition and virulence in Mycobacterium tuberculosis. J Bacteriol 188: 424-430. PMI: 16385031
Rodriguez GM (2006) Control of iron metabolism in Mycobacterium tuberculosis. Trends Microbiol 14: 320-327. PMI: 16759864
Rodriguez GM, Smith I (2004) Iron Metabolism in Pathogenic Bacteria. In Crosa J and Payne S (eds.), Iron transport in Bacteria: Molecular Genetics, Biochemistry, Microbial Pathogenesis and Ecology. ASM Press.
Rodriguez GM, Smith I (2003) Mechanisms of iron regulation in mycobacteria: role in physiology and virulence. Mol Microbiol 47: 1485-1494. PMI: 12622807
Rodriguez GM, Voskuil MI, Gold B, Schoolnik GK, Smith I (2002) ideR, An essential gene in mycobacterium tuberculosis: role of IdeR in iron-dependent gene expression, iron metabolism, and oxidative stress response. Infect Immun 70: 3371-3381. PMI: 12065475
Gold B, Rodriguez GM, Marras SA, Pentecost M, Smith I (2001) The Mycobacterium tuberculosis IdeR is a dual functional regulator that controls transcription of genes involved in iron acquisition, iron storage and survival in macrophages. Mol Microbiol 42: 851-865. PMI: 11722747
Rodriguez GM, Gold B, Gomez M, Dussurget O, Smith I (1999) Identification and characterization of two divergently transcribed iron regulated genes in Mycobacterium tuberculosis. Tuber Lung Dis 79: 287-298. PMI: 10707257